
On heating above 250 ☌, the completely anhydrous form called β-anhydrite or "natural" anhydrite is formed. γ-Anhydrite reacts slowly with water to return to the dihydrate state, a property exploited in some commercial desiccants. Wikipedia explains (Ref 3): "On heating to 180 ☌ (356 ☏), the nearly water-free form, called γ-anhydrite (CaSO4 There are three well-defined hydrates of CaSO $_4$: the dihydrate, the hemihydrate (Plaster of Paris) and the anhydrous material, which can be especially useful (i.e., reactive) when not quite totally anhydrous. High temperatures can alter the crystal structure and render the desiccants permanently inactive." Care should be taken not to overheat DRIERITE Desiccants. Lower temperatures, regardless of heating time, will not regenerate DRIERITE unless applied under vacuum (26" Hg, 325° F or 28" Hg, 275° F). Temperatures in the range of 400° - 450° F are required to break these bonds and release absorbed moisture.

Absorbed moisture is water of hydration and is chemically bound to the calcium sulfate of DRIERITE. "Drierite can be regenerated by spreading the granules in a tray and heating them in an oven at about 425☏ (220☌) for 1 to 1.5 hours."Ī more informative website (Ref 2) explains "The temperature at which DRIERITE desiccants are regenerated is crucial in restoring DRIERITE to its original condition.
It is blue when active and pink when hydrated (Ref 1).
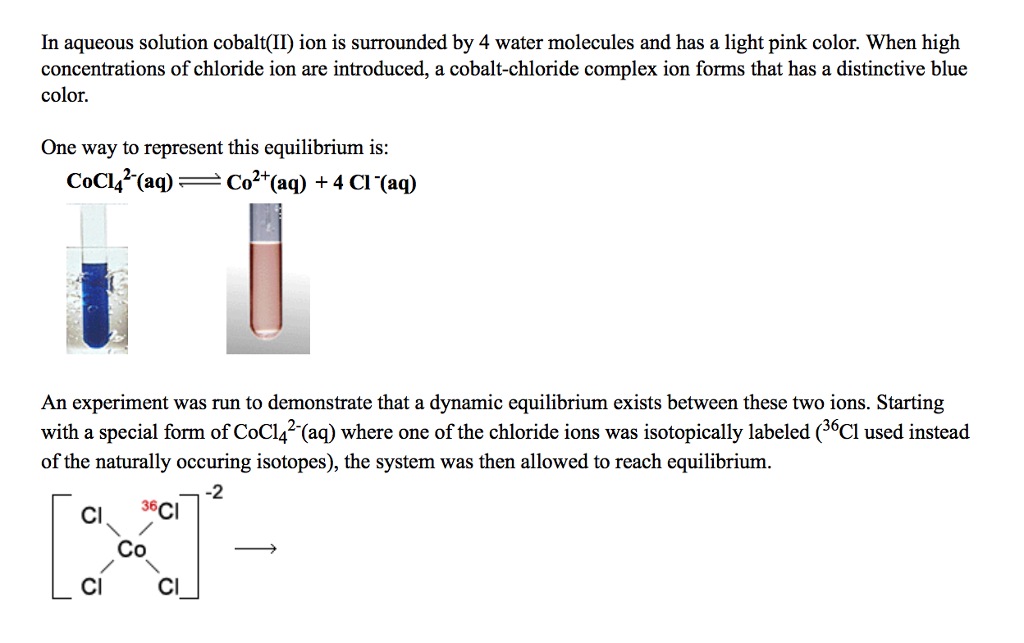
One of the "various forms" of cobalt chloride is Drierite, a mixture of cobalt chloride and calcium sulfate which is a common dessicant.


 0 kommentar(er)
0 kommentar(er)
